Cerebral cortex
| Brain: Cerebral cortex | ||
|---|---|---|
 |
||
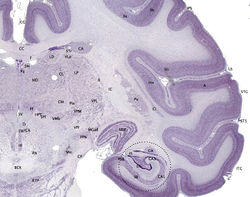
| The cerebral cortex is the outer layer depicted in dark violet. |
||
 |
||
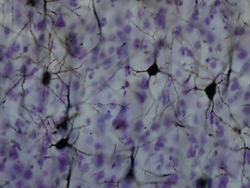
| Golgi-stained neurons in the cortex. | ||
| Latin | cortex cerebri | |
| Part of | Telencephalon | |
| NeuroLex ID | birnlex_1494 | |
The cerebral cortex is a sheet of neural tissue that is outermost to the cerebrum of the mammalian brain. It plays a key role in memory, attention, perceptual awareness, thought, language, and consciousness. It is constituted of up to six horizontal layers, each of which has a different composition in terms of neurons and connectivity. The human cerebral cortex is 2–4 mm (0.08–0.16 inches) thick.
In preserved brains, it has a gray color, hence the name "gray matter". In contrast to gray matter that is formed from neurons and their unmyelinated fibers, the white matter below them is formed predominantly by myelinated axons interconnecting neurons in different regions of the cerebral cortex with each other and neurons in other parts of the central nervous system.
The surface of the cerebral cortex is folded in large mammals, such that more than two-thirds of it in the human brains is buried in the grooves, called "sulci". The phylogenetically most recent part of the cerebral cortex, the neocortex (also called isocortex), is differentiated into six horizontal layers; the more ancient part of the cerebral cortex, the hippocampus (also called archicortex), has at most three cellular layers, and is divided into subfields. Neurons in various layers connect vertically to form small microcircuits, called columns. Different neocortical architectonic fields are distinguished upon variations in the thickness of these layers, their predominant cell type and other factors such as neurochemical markers.
Contents |
Development
The cerebral cortex develops from the most anterior part of the neural plate, a specialized part of the embryonihic ectoderm. The neural plate folds and closes to form the neural tube. From the cavity inside the neural tube develops the ventricular system, and, from the epithelial cells of its walls, the neurons and glia of the nervous system. The most anterior (frontal) part of the neural tube, the telencephalon, gives rise to the cerebral hemispheres and cortex.
Cortical neurons are generated within the ventricular zone, next to the ventricles. At first, this zone contains "progenitor" cells, which divide to produce glial and neuronal cells [1]. The glial fibers produced in the first divisions of the progenitor cells are radially oriented, spanning the thickness of the cortex from the ventricular zone to the outer, pial surface, and provide scaffolding for the migration of neurons outwards from the ventricular zone. The first divisions of the progenitor cells are symmetric, which duplicates the total number of progenitor cells at each mitotic cycle. Then, some progenitor cells begin to divide asymmetrically, producing one postmitotic cell that migrates along the radial glial fibers, leaving the ventricular zone, and one progenitor cell, which continues to divide until the end of development, when it differentiates into a glial cell or an ependymal cell. The migrating daughter cells become the pyramidal neurons of the cerebral cortex.[2]
The layered structure of the mature cerebral cortex is formed during development. The first pyramidal neurons generated migrate out of the ventricular zone and subventricular zone, together with Cajal-Retzius cells from the preplate. Next, a cohort of neurons migrating into the middle of the preplate divides this transient layer into the superficial marginal zone, which will become layer one of the mature neocortex, and the subplate, forming a middle layer called the cortical plate. These cells will form the deep layers of the mature cortex, layers five and six. Later born neurons migrate radially into the cortical plate past the deep layer neurons, and become the upper layers (two to four). Thus, the layers of the cortex are created in an inside-out order. The only exception to this inside-out sequence of neurogenesis occurs in the layer I of primates, in which, contrary to rodents, neurogenesis continues throughout the entire period of corticogenesis.[3]
Evolution
The cerebral cortex is derived from the pallium, a layered structure found in the forebrains of all vertebrates. The basic form of the pallium is a cylindrical layer enclosing fluid-filled ventricles. Around the circumference of the cylinder are four zones, the dorsal pallium, medial pallium, ventral pallium, and lateral pallium, which are thought respectively to give rise to the neocortex, hippocampus, amygdala, and olfactory cortex.
Until recently, no counterpart to the cerebral cortex has been recognized in invertebrates. However, a study published in the journal Cell in 2010, based on gene expression profiles, reported strong affinities between the cerebral cortex and the mushroom bodies of ragworms.[4] Mushroom bodies are structures in the brains of many types of worms and arthropods that are known to play important roles in learning and memory; the genetic evidence indicates a common evolutionary origin, and therefore indicates that the origins of the earliest precursors of the cerebral cortex date back to the early Precambrian era.
Laminar pattern
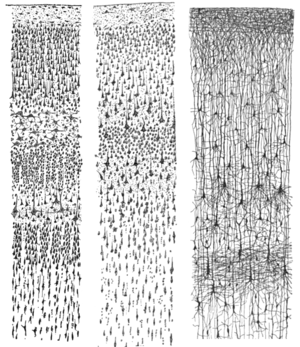
The different cortical layers each contain a characteristic distribution of neuronal cell types and connections with other cortical and subcortical regions. One of the clearest examples of cortical layering is the Stria of Gennari in the primary visual cortex. This is a band of whiter tissue that can be observed with the naked eye in the fundus of the calcarine sulcus of the occipital lobe. The Stria of Gennari is composed of axons bringing visual information from the thalamus into layer four of visual cortex.
Staining cross-sections of the cortex to reveal the position of neuronal cell bodies and the intracortical axon tracts allowed neuroanatomists in the early 20th century to produce a detailed description of the laminar structure of the cortex in different species. After the work of Korbinian Brodmann (1909), the neurons of the cerebral cortex are grouped into six main layers, from outside (pial surface) to inside (white matter):
- The molecular layer I, which contains few scattered neurons and consists mainly of extensions of apical dendritic tufts of pyramidal neurons and horizontally-oriented axons, as well as glial cells[5]. Some Cajal-Retzius and spiny stellate neurons can be found here. Inputs to the apical tufts are thought to be crucial for the ‘‘feedback’’ interactions in the cerebral cortex involved in associative learning and attention.[6] While it was once thought that the input to layer I came from the cortex itself,[7] it is now realized that layer I across the cerebral cortex mantle receives substantial input from ‘‘matrix’’ or M-type thalamus cells[8] (in contrast to ‘‘core’’ or C-type that go to layer IV).[9]
- The external granular layer II, which contains small pyramidal neurons and numerous stellate neurons
- The external pyramidal layer III, which contains predominantly small and medium-size pyramidal neurons, as well as non-pyramidal neurons with vertically-oriented intracortical axons; layers I through III are the main target of interhemispheric corticocortical afferents, and layer III is the principal source of corticocortical efferents
- The internal granular layer IV, which contains different types of stellate and pyramidal neurons, and is the main target of thalamocortical afferents from thalamus type C neurons.[9] as well as intra-hemispheric corticocortical afferents
- The internal pyramidal layer V, which contains large pyramidal neurons (such as the Betz cells in the primary motor cortex); it is the principal source of subcortical efferents
- The multiform layer VI, which contains few large pyramidal neurons and many small spindle-like pyramidal and multiform neurons; layer VI sends efferent fibers to the thalamus, establishing a very precise reciprocal interconnection between the cortex and the thalamus.[10] These connections are both excitatory and inhibitory. Neurons send excitatory fibers to neurons in the thalamus and also from collateral to them ones via the thalamic reticular nucleus that inhibit these thalamus neurons or ones adjacent to them.[11] Since the inhibitory output is reduced by cholinergic input to the cerebral cortex, this provides the brainstem with adjustable "gain control for the relay of lemnsical inputs".[11]
It is important to note that the cortical layers are not simply stacked one over the other; there exist characteristic connections between different layers and neuronal types, which span all the thickness of the cortex. These cortical microcircuits are grouped into cortical columns and minicolumns, the latter of which have been proposed to be the basic functional units of cortex.[12] In 1957, Vernon Mountcastle showed that the functional properties of the cortex change abruptly between laterally adjacent points; however, they are continuous in the direction perpendicular to the surface. Later works have provided evidence of the presence of functionally distinct cortical columns in the visual cortex (Hubel and Wiesel, 1959),[13] auditory cortex and associative cortex.
Cortical areas that lack a layer IV are called agranular. Cortical areas that have only a rudimentary layer IV are called dysgranular[14]. Information processing within each layer is determined by different temporal dynamics with that in the layers II/III having a slow 2Hz oscillation while that in layer V having a fast 10–15 Hz one.[15]
Connections of the cerebral cortex
The cerebral cortex is connected to various subcortical structures such as the thalamus and the basal ganglia, sending information to them along efferent connections and receiving information from them via afferent connections. Most sensory information is routed to the cerebral cortex via the thalamus. Olfactory information, however, passes through the olfactory bulb to the olfactory cortex (piriform cortex). The vast majority of connections are from one area of the cortex to another rather than to subcortical areas; Braitenberg and Schüz (1991) put the figure as high as 99%.[16]
The cortex is commonly described as comprising three parts: sensory, motor, and association areas.
Sensory areas
The sensory areas are the areas that receive and process information from the senses. Parts of the cortex that receive sensory inputs from the thalamus are called primary sensory areas. The senses of vision, audition, and touch are served by the primary visual cortex, primary auditory cortex and primary somatosensory cortex. In general, the two hemispheres receive information from the opposite (contralateral) side of the body. For example the right primary somatosensory cortex receives information from the left limbs, and the right visual cortex receives information from the left visual field. The organization of sensory maps in the cortex reflects that of the corresponding sensing organ, in what is known as a topographic map. Neighboring points in the primary visual cortex, for example, correspond to neighboring points in the retina. This topographic map is called a retinotopic map. In the same way, there exists a tonotopic map in the primary auditory cortex and a somatotopic map in the primary sensory cortex. This last topographic map of the body onto the posterior central gyrus has been illustrated as a deformed human representation, the somatosensory homunculus, where the size of different body parts reflects the relative density of their innervation. Areas with lots of sensory innervation, such as the fingertips and the lips, require more cortical area to process finer sensation.
Motor areas
The motor areas are located in both hemispheres of the cortex. They are shaped like a pair of headphones stretching from ear to ear. The motor areas are very closely related to the control of voluntary movements, especially fine fragmented movements performed by the hand. The right half of the motor area controls the left side of the body, and vice versa.
Two areas of the cortex are commonly referred to as motor:
- Primary motor cortex, which executes voluntary movements
- Supplementary motor areas and premotor cortex, which select voluntary movements.
In addition, motor functions have been described for:
- Posterior parietal cortex, which guides voluntary movements in space
- Dorsolateral prefrontal cortex, which decides which voluntary movements to make according to higher-order instructions, rules, and self-generated thoughts.
Association areas
Association areas function to produce a meaningful perceptual experience of the world, enable us to interact effectively, and support abstract thinking and language. The parietal, temporal, and occipital lobes - all located in the posterior part of the cortex - organize sensory information into a coherent perceptual model of our environment centered on our body image. The frontal lobe or prefrontal association complex is involved in planning actions and movement, as well as abstract thought. In the past it was theorized that language abilities are localized in the left hemisphere in areas 44/45, the Broca's area, for language expression and area 22, the Wernicke's area, for language reception. However, language is no longer limited to easily identifiable areas. More recent research suggests that the processes of language expression and reception occur in areas other than just the perisylvian structures, such as the prefrontal lobe, basal ganglia, cerebellum, pons, caudate nucleus, and others. [17]
Association areas Definition: areas of the cerebal cortex that are not involved in primary motor or sensory functions; rather, they are involved in higher mental functions such as learning, remembering, thinking, and speaking. Author:David G Meyers Publisher: Catherine Woods Title: Exploring Psychology seventh edition pages: 55
Classification
Based on the differences in lamination the cerebral cortex can be classified into two major groups:
- Isocortex (homotypical cortex), the part of the cortex with six layers.
- Allocortex (heterotypical cortex) the part of the cortex with less than six layers (varying in number). The allocortex includes the olfactory cortex and the hippocampus.
Auxiliary classes are:
- Mesocortex, classification between isocortex and allocortex where layers 2, 3, and 4 are merged
- Proisocortex, Brodmann areas 24, 25, 32
- Periallocortex, comprising cortical areas adjacent to allocortex.
Based on supposed developmental differences the following classification also appears:
- Neocortex or Neopallium, which corresponds to the isocortex.
- Archicortex, which phylogenetically is the oldest cortex
- Paleocortex
In addition, cortex may be classified on the basis of gross topographical conventions into four lobes:
- Temporal Cortex
- Occipital Cortex
- Parietal Cortex
- Frontal Cortex
Cortical thickness
With magnetic resonance brain scanners, it is possible to get a measure for the thickness of the human cerebral cortex and relate it to other measures. The thickness of different cortical areas varies. In general, sensory cortex is thinner and motor cortex is thicker.[18] One study has found some positive association between the cortical thickness and intelligence.[19] Another study has found that the somatosensory cortex is thicker in migraine sufferers.[20]
See also
- Cortical column
- Frontal lobe
- Limbic system
- List of regions in the human brain
- Microgyrus
- Occipital lobe
- Parietal lobe
- Temporal lobe
- Cerebral hemisphere
- Neocortex
- Subplate
- Brain-computer interface
References
- ↑ Stephen C. Noctor, Alexander C. Flint, Tanily A. Weissman, Ryan S. Dammerman & Arnold R. Kriegstein (2001). "Neurons derived from radial glial cells establish radial units in neocortex". Nature 409 (6821): 714–720. doi:10.1038/35055553. PMID 11217860. http://www.nature.com/nature/journal/v409/n6821/abs/409714a0.html.
- ↑ P. Rakic (1988). "Specification of cerebral cortical areas". Science 241 (4862): 170–176. doi:10.1126/science.3291116. PMID 3291116. http://www.sciencemag.org/cgi/content/abstract/241/4862/170.
- ↑ Zecevic N, Rakic P (2001). "Development of layer I neurons in the primate cerebral cortex". J Neurosci. 21 (15): 5607–19. PMID 11466432. http://www.jneurosci.org/cgi/content/full/21/15/5607.
- ↑ Tomer R, Denes AS, Tessmar-Raible K, Arendt D (2010). "Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium". Cell 142: 800-809. doi:10.1016/j.cell.2010.07.043.
- ↑ Shipp, Stewart (2007-06-17). "Structure and function of the cerebral cortex". Current Biology 17 (12): R443–9. doi:10.1016/j.cub.2007.03.044. PMID 17580069. http://www.cell.com/current-biology/retrieve/pii/S0960982207011487. Retrieved 2009-02-17.
- ↑ Gilbert CD, Sigman M. (2007). Brain states: top-down influences in sensory processing. Neuron. 54(5):677-96. PMID 175534
- ↑ Cauller L. (1995). Layer I of primary sensory neocortex: where top-down converges upon bottom-up. Behav Brain Res. 71(1-2):163-70. PMID 8747184
- ↑ Rubio-Garrido P, Pérez-de-Manzo F, Porrero C, Galazo MJ, Clascá F. (2009). Thalamic input to distal apical dendrites in neocortical layer 1 is massive and highly convergent. Cereb Cortex. 19(10):2380-95. PMID 19188274 doi:10.1093/cercor/bhn259
- ↑ 9.0 9.1 Jones EG. (1998). Viewpoint: the core and matrix of thalamic organization. Neuroscience. 85(2):331-45. PMID 9622234
- ↑ Creutzfeldt, O. 1995. Cortex Cerebri. Springer-Verlag.
- ↑ 11.0 11.1 Lam YW, Sherman SM. (2010). Functional Organization of the Somatosensory Cortical Layer 6 Feedback to the Thalamus. Cereb Cortex. 20:13-24. doi:10.1093/cercor/bhp077 PMID 19447861
- ↑ Mountcastle, V. 1997. The columnar organization of the neocortex. Brain, 120:701-722
- ↑ HUBEL DH, WIESEL TN (October 1959). "Receptive fields of single neurones in the cat's striate cortex". J. Physiol. (Lond.) 148: 574–91. PMID 14403679. PMC 1363130. http://www.jphysiol.org/cgi/pmidlookup?view=long&pmid=14403679.
- ↑ S.M. Dombrowski , C.C. Hilgetag , and H. Barbas. Quantitative Architecture Distinguishes Prefrontal Cortical Systems in the Rhesus Monkey.Cereb. Cortex 11: 975-988. "...they either lack (agranular) or have only a rudimentary granular layer IV (dysgranular)."
- ↑ Sun W, Dan Y. (2009). Layer-specific network oscillation and spatiotemporal receptive field in the visual cortex. Proc Natl Acad Sci U S A. 106:17986–17991 doi:10.1073/pnas.0903962106 PMID 19805197
- ↑ Braitenberg, V and Schüz, A 1991. "Anatomy of the Cortex: Statistics and Geometry" NY: Springer-Verlag
- ↑ Cathy J. Price (2000). "The anatomy of language: contributions from functional neuroimaging". Anatomy 197 (3): 335–359. doi:10.1046/j.1469-7580.2000.19730335.x.
- ↑ Frithjof Kruggel, Martina K. Brückner, Thomas Arendt, Christopher J. Wiggins and D. Yves von Cramon (2003). "Analysing the neocortical fine-structure". Medical Image Analysis 7 (3): 251–264. doi:10.1016/S1361-8415(03)00006-9.
- ↑ Katherine L. Narr, Roger P. Woods, Paul M. Thompson, Philip Szeszko, Delbert Robinson, Teodora Dimtcheva, Mala Gurbani, Arthur W. Toga and Robert M. Bilder (2007). "Relationships between IQ and Regional Cortical Grey Matter Thickness in Healthy Adults". Cerebral Cortex 17 (9): 2163–2171. doi:10.1093/cercor/bhl125. PMID 17118969.
- ↑ Alexandre F.M. DaSilva, Cristina Granziera, Josh Snyder and Nouchine Hadjikhani (2007). "Thickening in the somatosensory cortex of patients with migraine". Neurology 69 (21): 1990–1995. doi:10.1212/01.wnl.0000291618.32247.2d. PMID 18025393. News report:
- Catharine Paddock (2007-11-20). "Migraine Sufferers Have Thicker Brain Cortex". Medical News Today. http://www.medicalnewstoday.com/articles/89286.php.
External links
- NeuroNames hier-20
- BrainMaps at UCDavis cerebral cortex
- Webvision - The primary visual cortex Comprehensive article about the structure and function of the primary visual cortex.
- Webvision - Basic cell types Image of the basic cell types of the monkey cerebral cortex.
- Development of the Cerebral Cortex Different topics on cortical development in the form of columns written by leading scientists.
- Cerebral Cortex - Cell Centered Database
- NIF Search - Cerebral Cortex via the Neuroscience Information Framework
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||